Research
My current research interests lie in understanding transport and thermodynamics in electrolyte solutions for energy storage applications. I use theoretical tools from statistical mechanics and nonequilibrium thermodynamics as well as atomistic simulation techniques such as molecular dynamics.
Transport in Li-Ion Battery Electrolytes
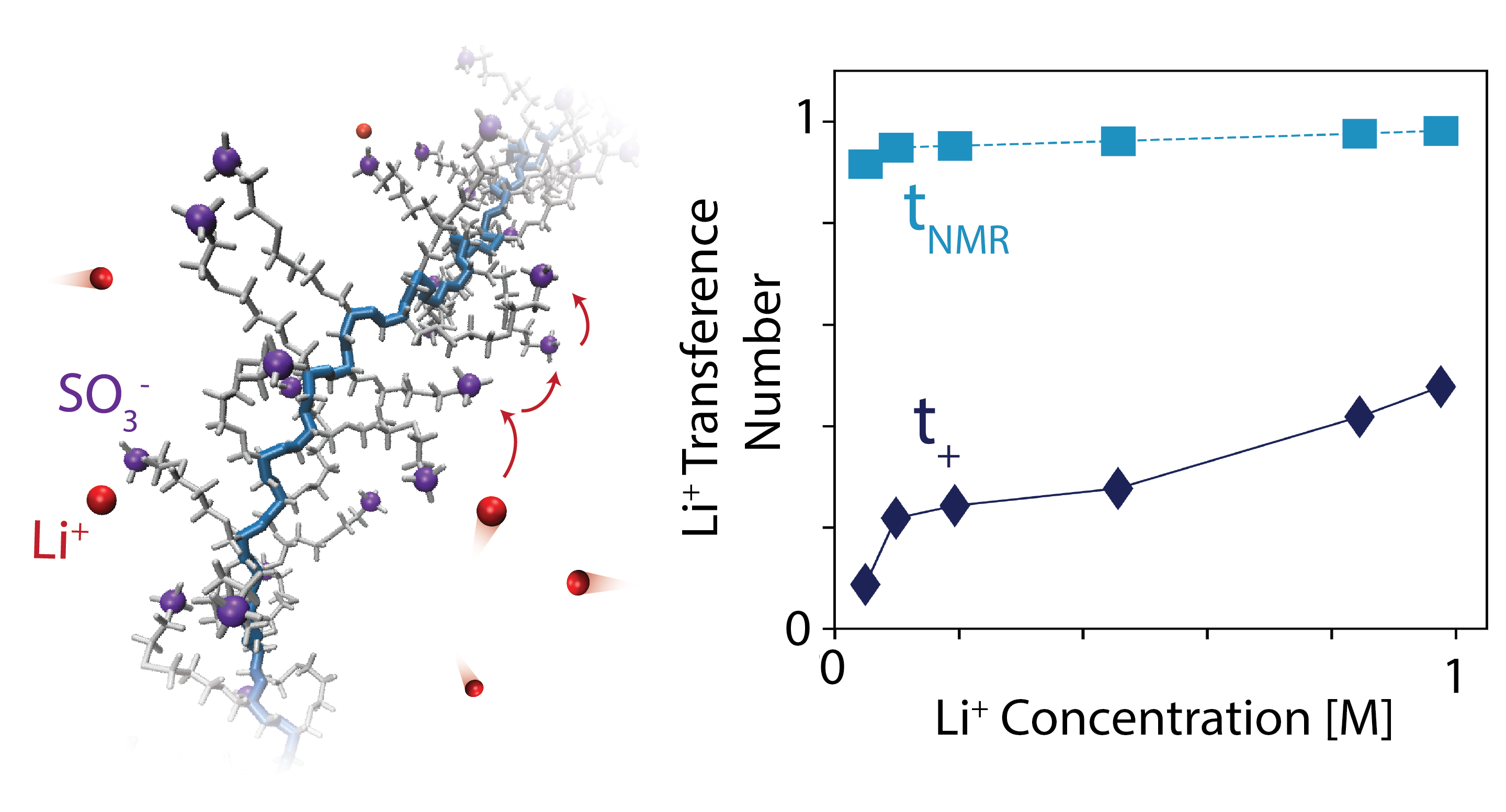
Li-ion battery electrolytes with enhanced transport properties may enable batteries with higher energy and power densities. I use molecular dynamics simulations to study how these macroscopic transport quantities arise from molecular-level interactions between ions in solution. Using the theoretical developments described below, I rigorously quantify ion correlations in the electrolyte, which can substantially impact macroscopic transport but are challenging to measure experimentally. The aim of this work is to provide insight into the types of electrolyte formulations which may yield high conductivity, high cation transference number, or facile transport under low temperature conditions. My work thus far has in particular explored the transport properties of nonaqueous polyelectrolyte solutions and polymerized ionic liquids.
K. D. Fong, J. Self, B. D. McCloskey, K. A. Persson. “Onsager Transport Coefficients and Transference Numbers in Polyelectrolyte Solutions and Polymerized Ionic Liquids.” Macromolecules, 2020, 53, 21: 9503-9512. [doi]
K. D. Fong, J. Self, K. M. Diederichsen, B. M. Wood, B. D. McCloskey, and K. A. Persson. “Ion Transport and the True Transference Number in Nonaqueous Polyelectrolyte Solutions for Lithium-Ion Batteries.” ACS Central Science, 2019, 5: 1250-1260. [doi]
Theory for Transport in Concentrated Electrolytes
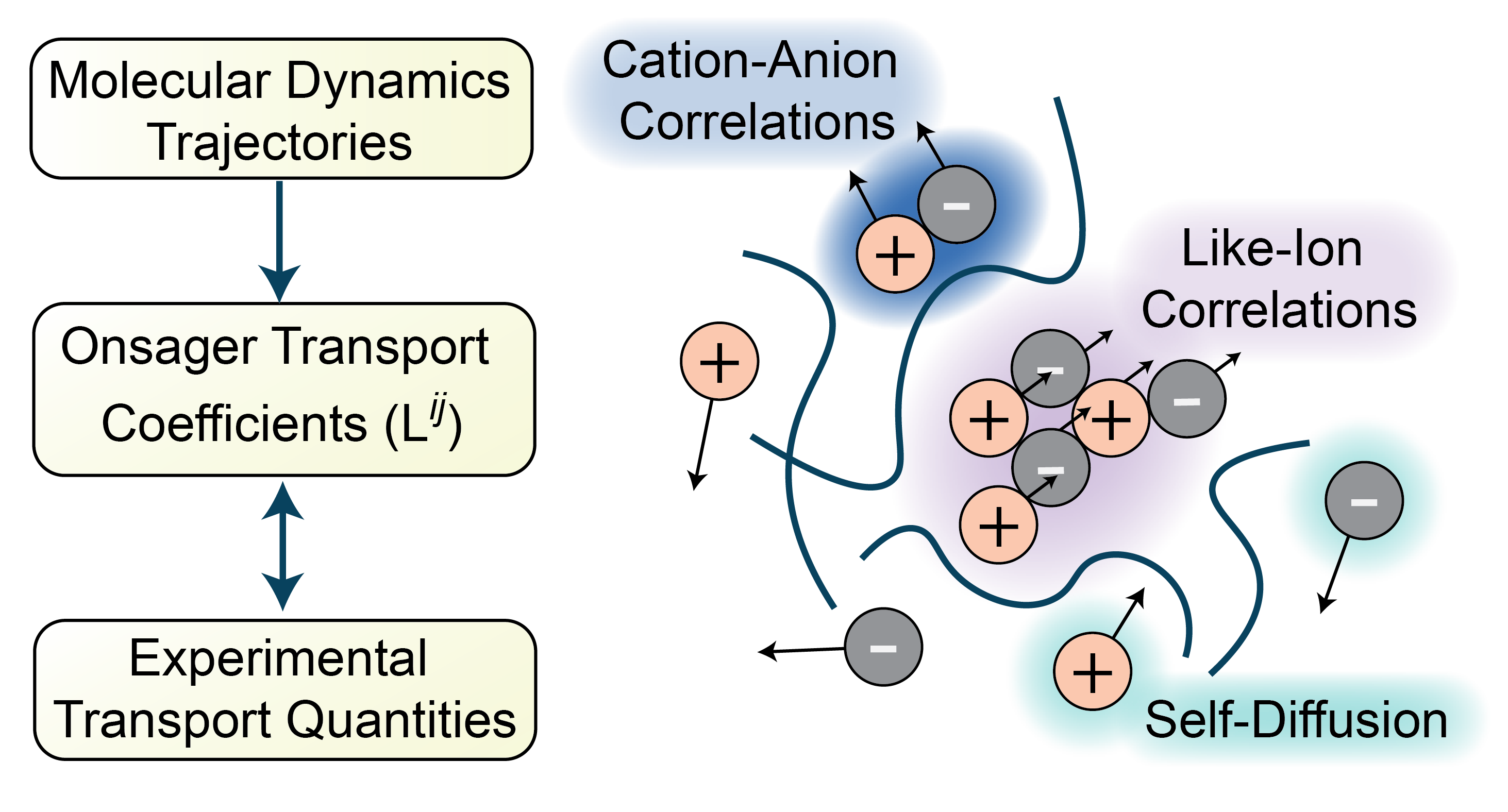
Our understanding of transport phenomena in electrolyte solutions should ideally persist across length scales: we desire both continuum-level insight into macroscopic concentration and electric potential profiles as well as a molecular-level understanding of the mechanisms governing ion motion. However, the most commonly used theories to describe continuum-level electrolyte transport, namely the Stefan-Maxwell equations, yield transport coefficients which lack clear physical interpretation at the atomistic level and cannot be easily computed from molecular simulations. I work on the theoretical development and application of the Onsager transport framework to analyze transport at both the continuum and molecular levels. This theory integrates continuum mechanics, nonequilibrium thermodynamics, and electromagnetism to give an expression for internal entropy production in electrolytes, yielding the Onsager transport equations: linear laws relating the electrochemical potential gradients and fluxes of each species in solution. At the atomistic level, the transport coefficients emerging from this theory directly quantify ion correlations in the electrolyte; these transport coefficients may be computed directly from molecular simulations using Green-Kubo relations derived from Onsager’s regression hypothesis. At the continuum level, the Onsager transport framework provides the governing equations for solving macroscopic boundary value problems in an electrochemical cell. This framework allows for rigorous analysis of transport across length scales in complex electrolyte solutions.
K. D. Fong, H. K. Bergstrom, B. D. McCloskey, K. K. Mandadapu. “Transport Phenomena in Electrolyte Solutions: Non-Equilibrium Thermodynamics and Statistical Mechanics.” AIChE Journal, 2020, 66, 12: e17091. [doi]
K. D. Fong, J. Self, B. D. McCloskey, K. A. Persson. “Ion Correlations and Their Impact on Transport in Polymer-Based Electrolytes.” Macromolecules, 2021, 54, 6: 2575-2591. [doi]
